Dibromofluoromethane (DBFM)
DBFM (Catalog # VCBFM, CAS # 1868-53-7, bp 65 °C) is a reagent for a wide variety of functionalizations - from cyclopropanation to alkylation to dipolar cycloadditions. In many of these transformations, the resultant products contain fluorine. Available on commercial scale.
Alternate Names: DBFM, fluorodibromomethane
Catalog #: VCDBFM
CAS #: 1868-53-7
Molecular Weight: 191.83 g/mol
Density: 2.42 g/mL
Boiling Point: 65°C
1H NMR Spectra (CDCl3): 7.71 (d, J = 48.3 Hz, 1H) ppm
13C NMR Spectra (CDCl3): 73.8 (d, J = 315.0 Hz, 1H) ppm
19F NMR Spectra (CDCl3): -84.08 (d, J = 48.9 Hz, 1H) ppm
Wikipedia Link: http://en.wikipedia.org/wiki/Dibromofluoromethane
Material Safety Data Sheet (MSDS) / SafetyData Sheet (SDS): [DOWNLOAD]
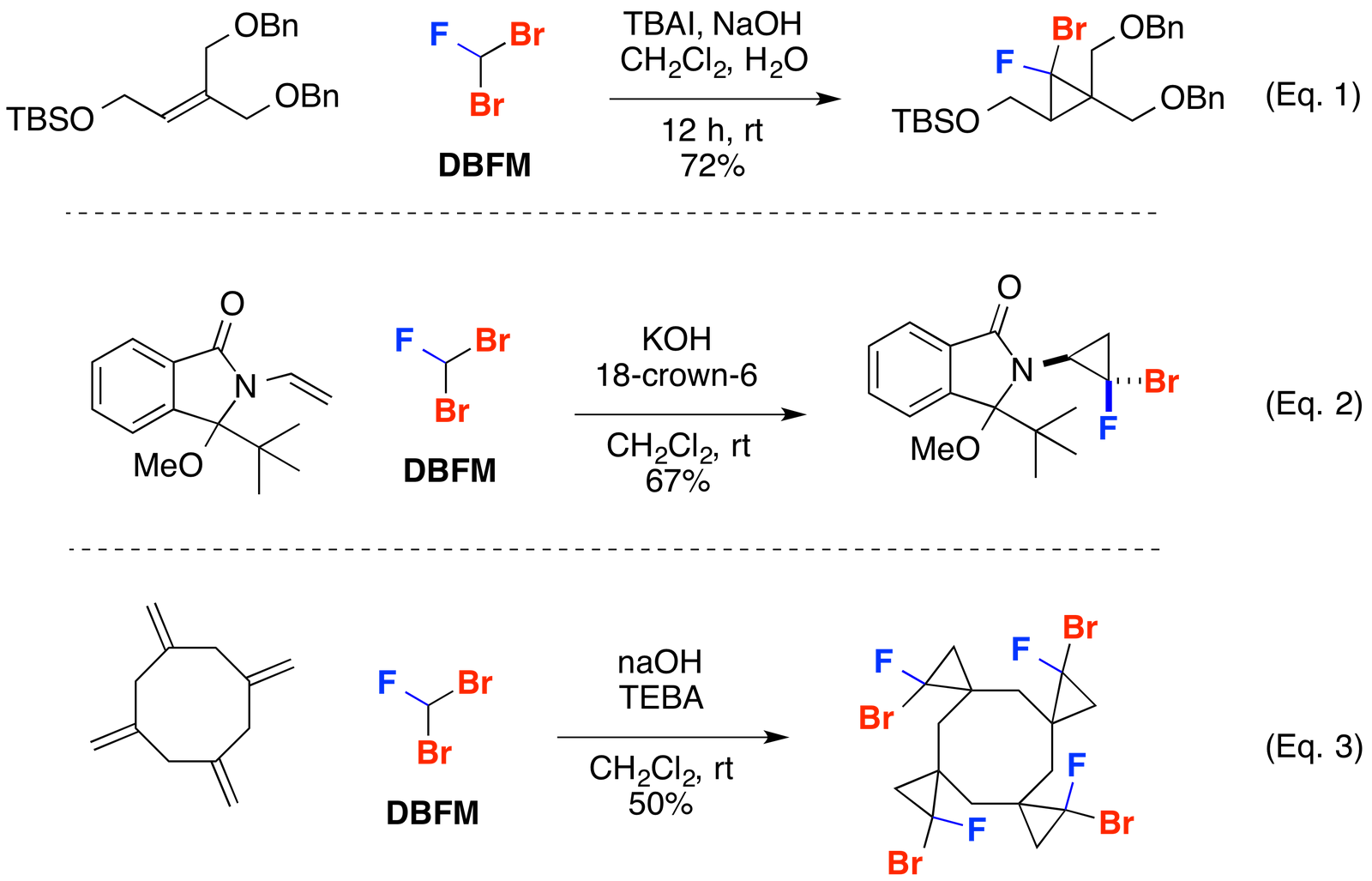
Cyclopropanation
Dibromofluoromethane (DBFM) has proven a powerful reagent for the cyclopropanation of alkenes (J. Org. Chem. 2009, 74, 7075-7083; Chem. Eur. J. 2009, 15, 11256-11265), (Eur. J. Org. Chem. 2010, 4145-4150), (J. Org. Chem. 2012, 77, 9893-9899), (Eq. 1-3). Zemlicka and co-workers demonstrated the cyclopropation of a trisubstituted alkene with DBFM to access fluoroanalogues of the anti-cytomegalovirus agent cyclopropavir (Nucleos. Nucleot. Nucl. 2007, 26, 231-243) (Eq. 1). Hayakawa and co-workers showcased the stereoselectively cyclopropanation of an enamine using dibromofluoromethane (Chem. Lett. 2004, 33, 464-465) (Eq. 2). Averina and co-workers employed DBFM in a tetracyclopropantion to access polyspirocycles (J. Org. Chem. 2014, 79, 8163-8170) (Eq. 3).
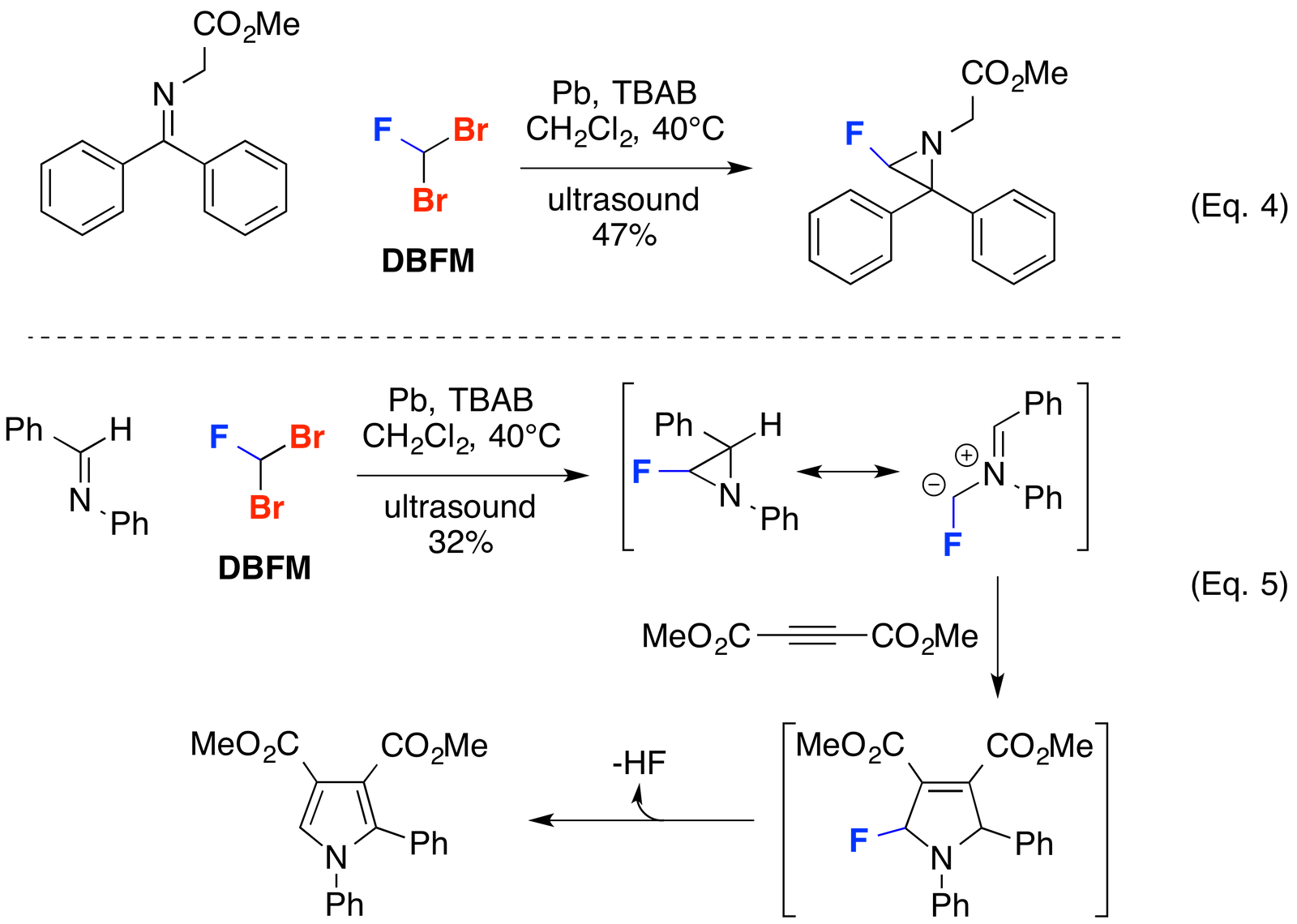
Aziridine Formation and Dipolar Cycloadditions
Khlebnikov and co-workers have exploited the carbene-like reactivity of dibromofluoromethane (DBFM) to access aziridines (Russ. J. Org. Chem. 2007, 43, 286-296) (Russ. J. Org. Chem. 2010, 46, 976-986) (Eq. 4). These aziradines have been shown to be useful for accessing azomethine ylides which undergo [3+2] dipolar cycloadditions with dipolarophiles to construct pyrroles (Tetrahedron Lett. 2005, 46, 8337-8340) (Eq. 5).
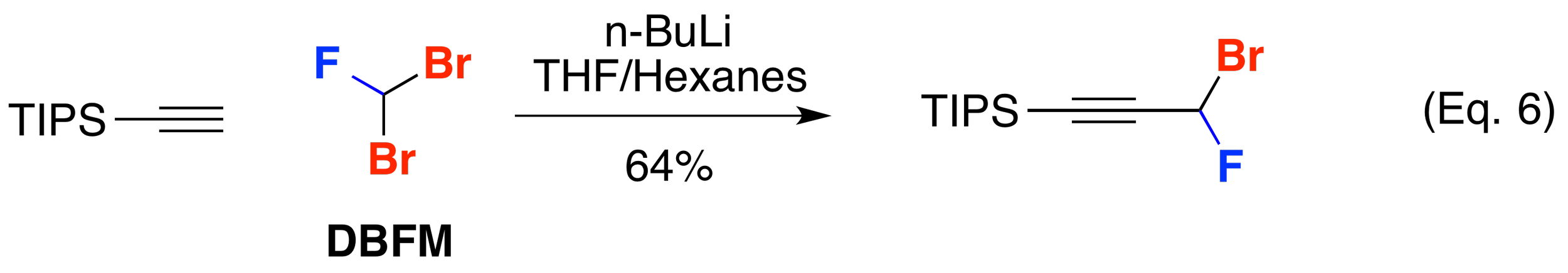
Alkylation by Nucleophiles
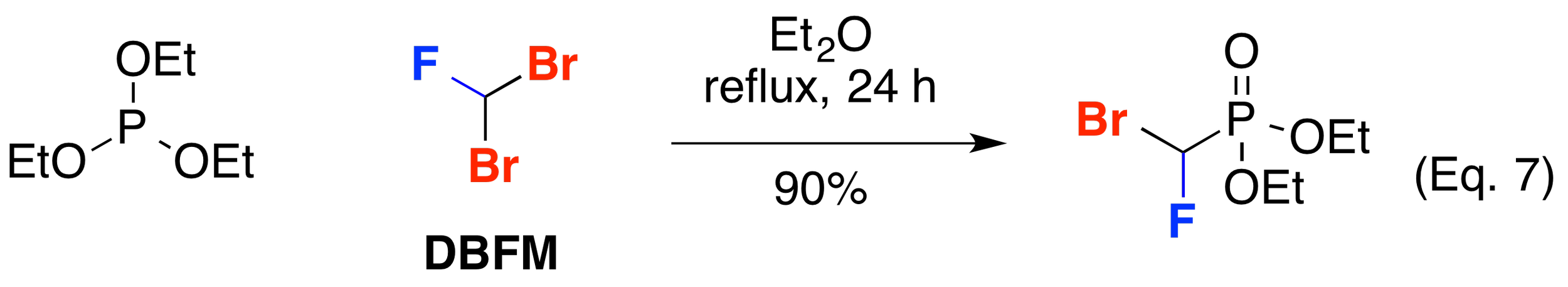
Hammond and co-workers has shown that dibromofluoromethane (DBFM) is a good electrophile for alkylation by lithiated acetylides such as TIPS acetylene (J. Org. Chem. 2000, 65, 4217-4221) (Org. Lett. 2002, 4, 2437-2439) (Eq. 6). Dibromofluoromethane (DBFM) has also been used in reactions with phosphorous. Poulter and co-workers exploited the reactivity of triethyl phosphite in an Arbuzov reaction with DBFM to make the corresponding phosphonate (J. Fluorine Chem. 1977, 10, 329-332) (Phosphorous, Sulfur, Silicon Relat. Elem. 1993, 75, 139-142) (J. Org. Chem. 1986, 51, 4768-4779) (Eq. 7). DBFM has also been employed in the synthesis of phosphinate hapetens to neutralize organophosphorus chemical weapons (Chem. Eur. J. 2000, 6, 1050-1063).