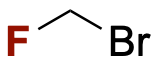
Bromofluoromethane (BFM)
BFM is an important building block primarily used in the commercial manufacture of the APIs fulticasone propionate and fuliticasone furoate. Using proprietary technology, Valliscor manufactures kilogram to commercial scale quantities of BFM for the pharmaceutical industry at its Corvallis, Oregon production facility.
Alternate Names: BFM, bromofluoromethylene, CFC 31B1, R 31B1
Catalog #: VCBFM
CAS #: 373-52-4
Molecular Weight: 112.916
Density: 1.76 g/mL
Boiling Point: 20°C
1H NMR Spectra (CDCl3): 6.1 (d, J(F-H) = 48 Hz, 2H) ppm
13C NMR Spectra (CDCl3): 77.4 (d, J(C-F) = 247 Hz) ppm
19F NMR Spectra (CDCl3): -163.5 (t, J = 48.9 Hz) ppm
IR Spectra: Mol. Phys. 2006, 104, 3187-3192
Microwave Spectra: J. Mol. Spec. 2007, 241, 112-115
WikipediaLink: http://en.wikipedia.org/wiki/Bromofluoromethane
Material Safety Data Sheet (MSDS) / Safety Data Sheet (SDS): [DOWNLOAD]
Downloadable Flyer on Valliscor’s Bromofluoromethane (BFM) Capabilities: [DOWNLOAD]
Fluticasone and Related Examples
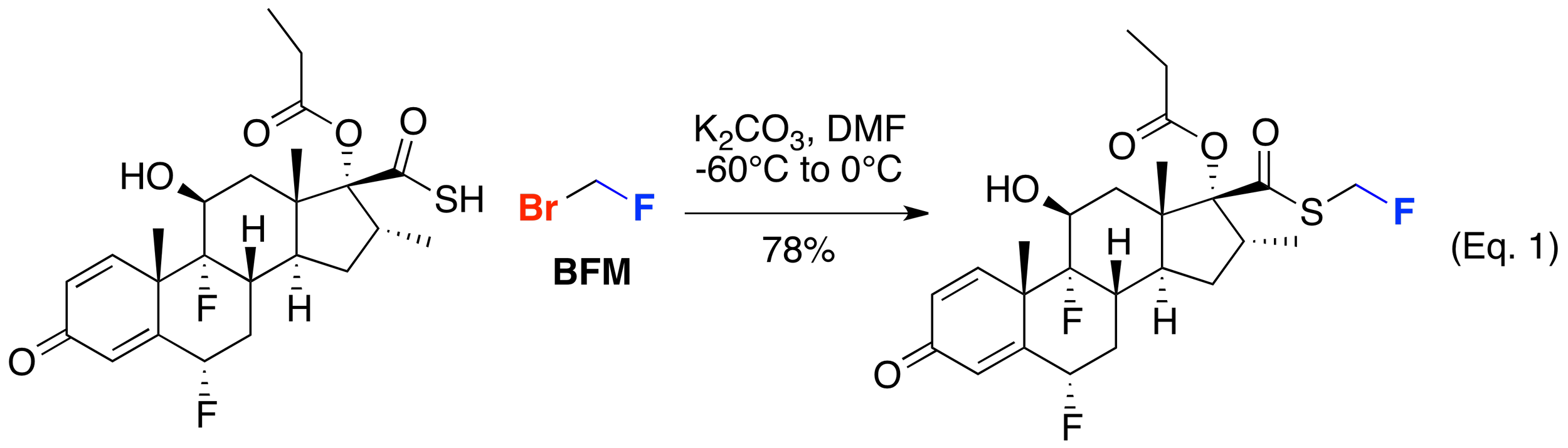
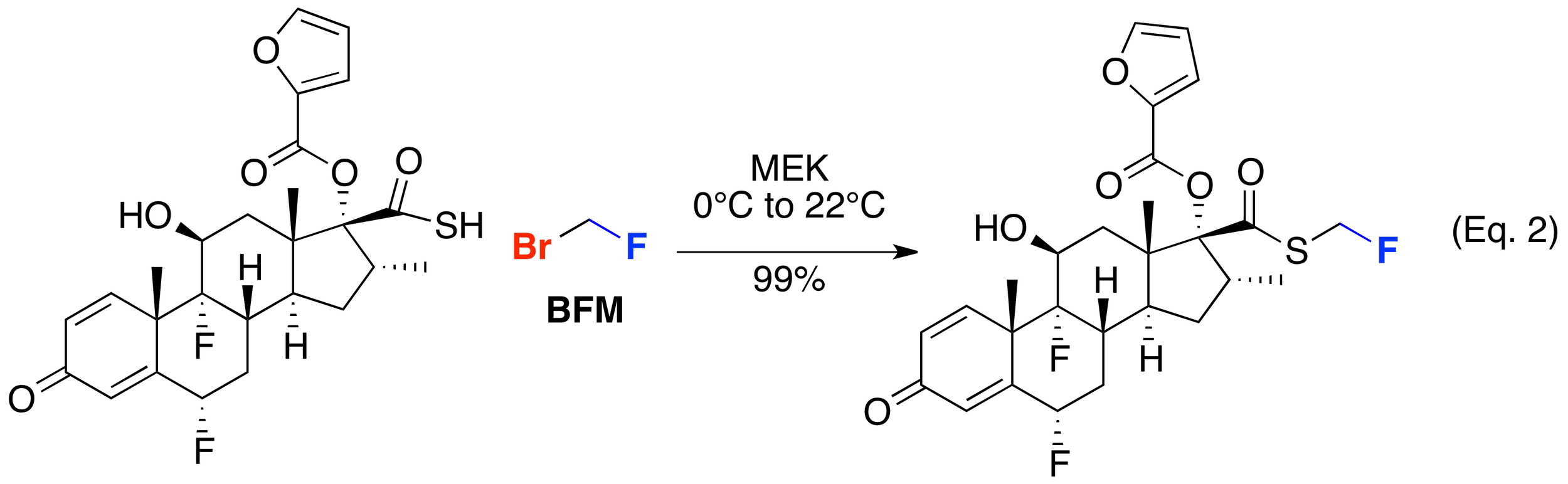
The fluoromethyl moiety has proven useful in a range of pharmaceutically relevant targets and agrochemically-significant compounds. One of the first examples of the use of bromofluoromethane in the synthesis of a commercial product is in the fluoromethylation of a thioacid to manufacture fluticasone propionate by Glaxo (Eq. 1). Subsequently, BFM has been shown to be generally useful in accessing other fluticasone-related molecules – including fluticasone furoate by GlaxoSmithKline (Eq. 2).Other companies have demonstrated the utility of BFM to construct related scaffolds including Chiesi Farmaceutici’s pyrrolidine-containing glucocortosteroids (Eq. 3) and biaryl ether-containing glucocorticoids from Pfizer (Eq. 4).
PET Imaging Examples
Bromofluoromethane (BFM) has also proven useful for accessing PET imaging molecules. BFM can be cross coupled with boronic esters to access fluoromethyl aromatic compounds (Eq. 5). Additionally, alkylation using phenolic nucleophiles such as the protected tyrosine derivative shown below provides a PET imaging precursor in good yield (Eq. 6).
Alkylation of Phenolic Compounds
Flouromethylation of phenols with bromofluoromethane (BFM) has proven useful to provide access to a variety of compounds. Acadia Pharmaceuticals reported the synthesis of cannabinoid CB2 receptor active compounds using precursors containing fluoromethyl ethers (Eq. 7). Astrazeneca synthesized oxabispidine compounds for treatment of cardiac arrhythmias derived from flouromethyl-alkylated aldehyde derivatives (Eq. 8).
Oxime Alkylation
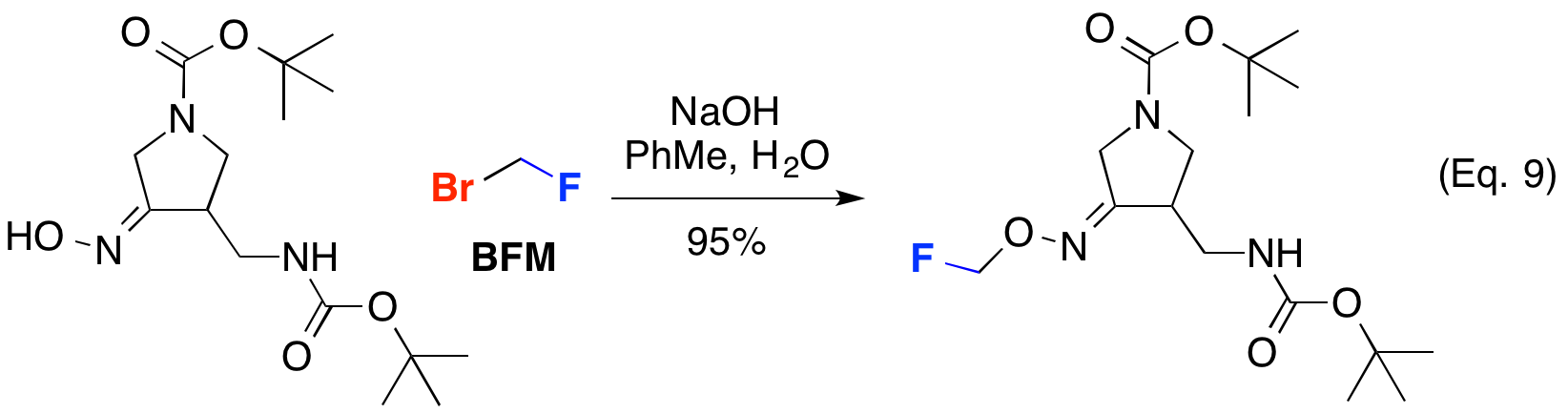
Bromofluoromethane (BFM) can be used to alkyl other oxygen nucleophiles including oximes as shown below (Eq. 9).
Enolate Alkylation
Bromofluoromethane (BFM) has also been employed for fluoromethylation of enolates. Takeda Pharmaceutical Company reported the alkylation of cyanoazetidine using LDA in good yield to access JAK inhibitors (Eq. 10).
Incorporation into Heterocylces
Bromofluoromethane (BFM) can also be utilized to alkylate complex heterocycles. Francotte and co-workers used BFM to construct new potentiators of AMPA receptors (Eq. 11). Sinochem utilized BFM to access the pyrazole derivative as potential broad spectrum insecticide (Eq. 12).
J. Med. Chem. 1994, 37, 3717-3729
PCT Int. Appl., WO2007114363 A2, 21 Dec 2027

PCT Int. Appl., WO2011095535 A2, 11 August 20211 (Eq. 3); PCT Int. App., WO201136940 A2, 2 Dec 2010
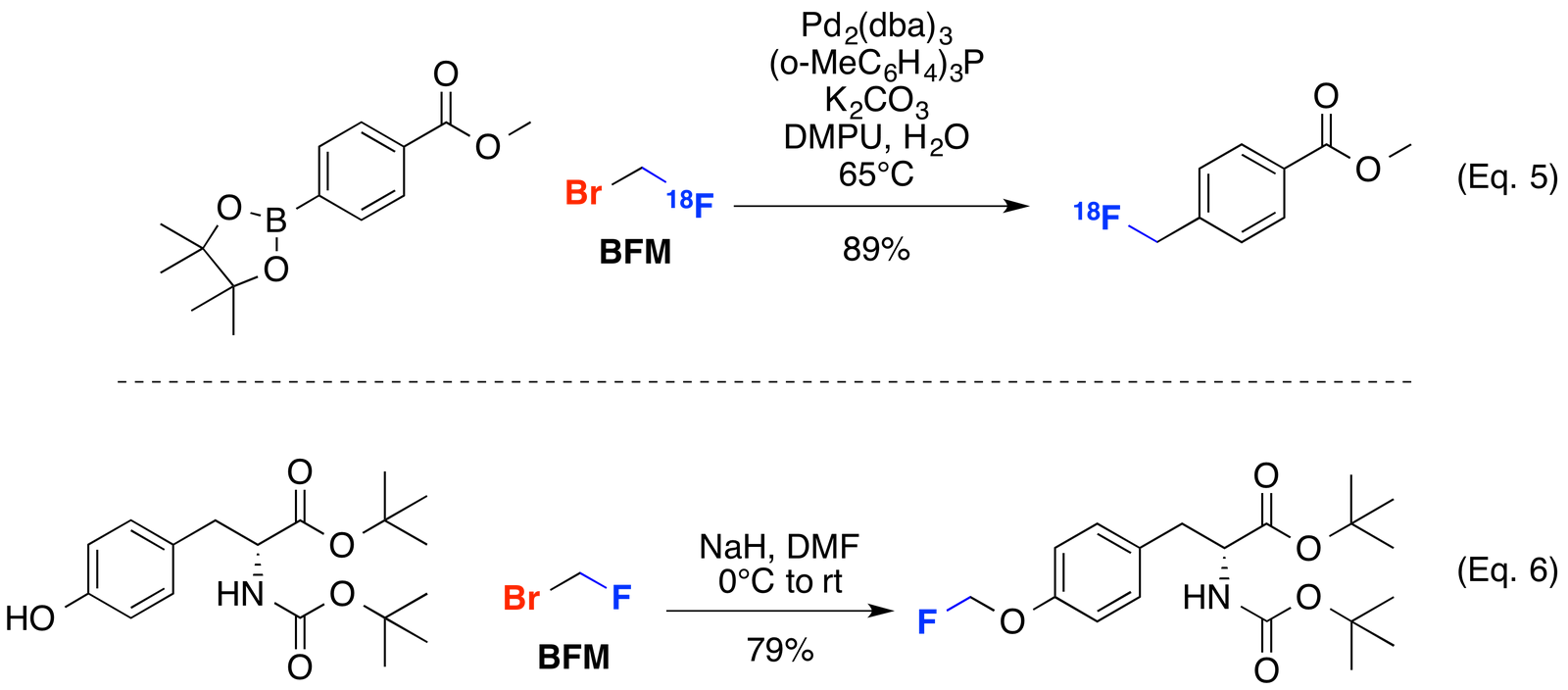
Bull. Chem. Soc. Jpn. 2012, 85, 1233-1238 (Eq. 5); PCT Int. Appl. WO2013001088, 3 Jan 2013) (Eq. 6).
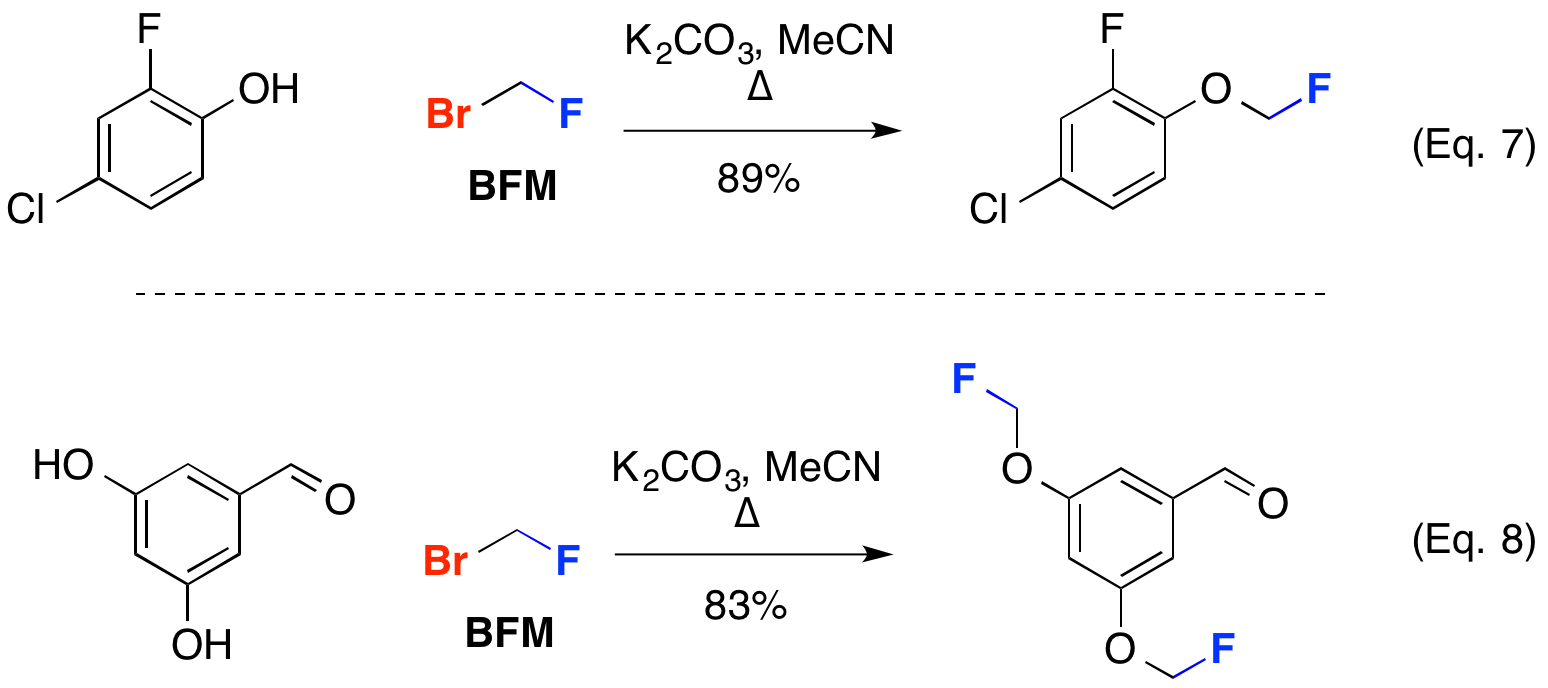
PCT Int. Appl. WO2008141249, 20 Nov 2008 (Eq. 7); PCT Int. Appl., WO2007069986 (Eq. 8)
Eur. J. Med. Chem. 2012, 47, 619-625.
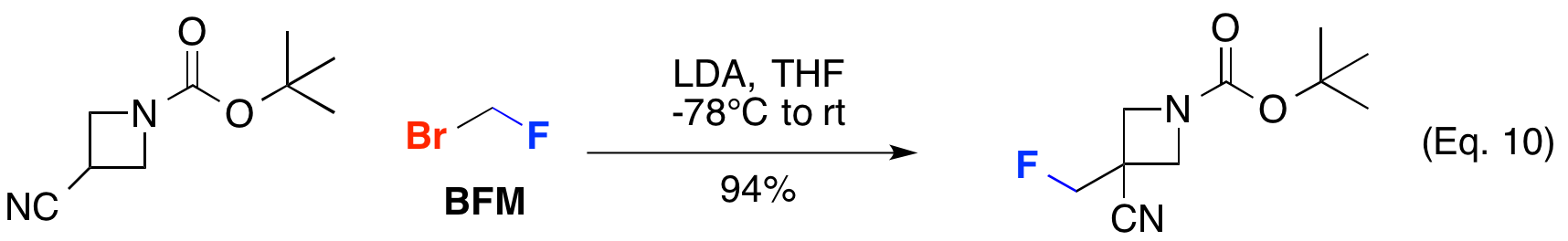
PCT Int. Appl., WO2010144486 A1, 16 Dec 2010
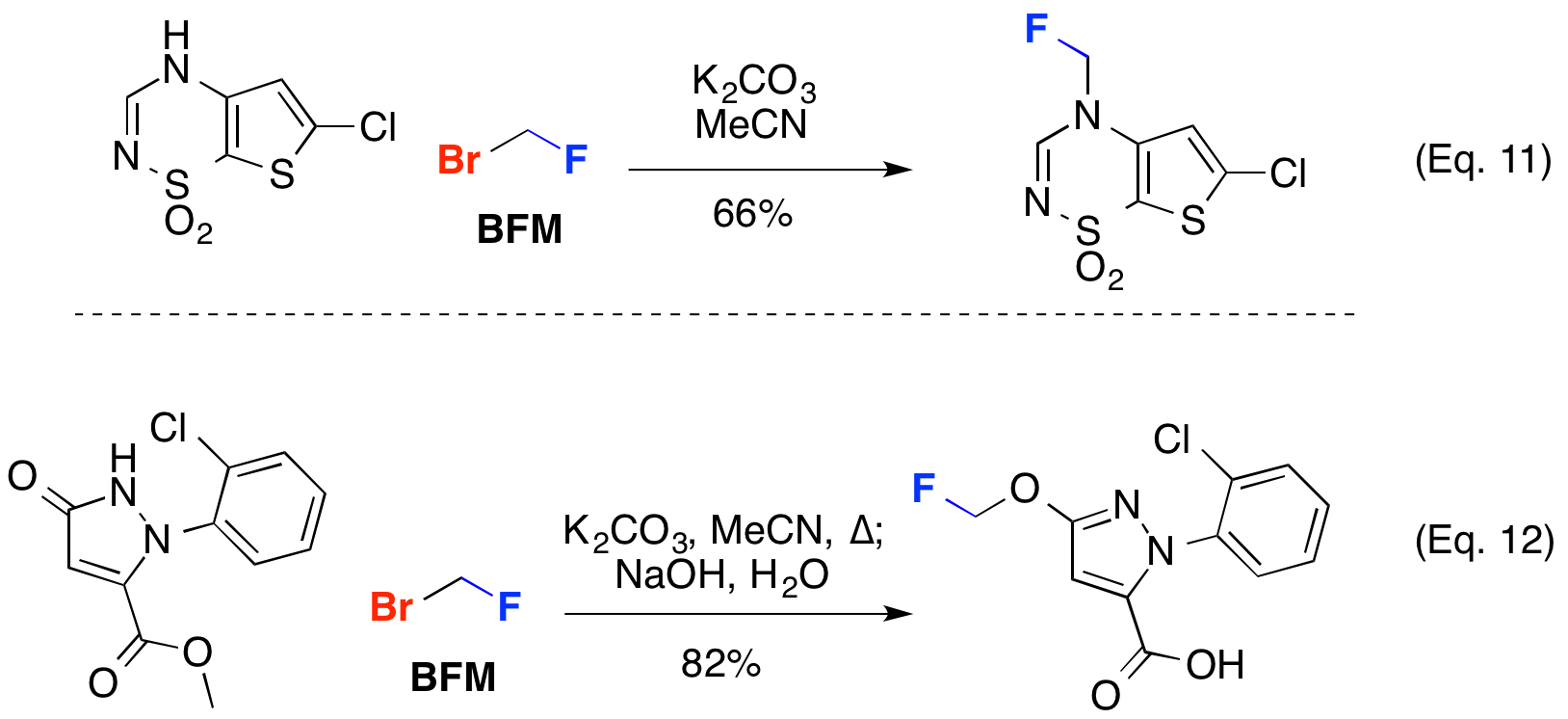
J. Med. Chem. 2013, 56, 7838-7860 (Eq. 11); PCT Int. Appl., WO2012034403 A1, 22 Mar 2012 (Eq. 12)